Biochemical Engineering Laboratory Research Projects
Research Interests
Biochemical engineering, Systems biology, Metabolic engineering, Biofilm physiology and control
Research Summary
Metabolic Network Analysis and Engineering
Life is comprised of thousands of chemical reactions organized into complex networks. These networks channel and transform nutrients acquired from the environment into products like work, heat, and new life. To understand the chemistries of life, the highly branched and highly coupled networks must be understood. We are utilizing a network analysis system known as elementary flux mode analysis to study the properties of microbial reaction networks. The method identifies every unique, mathematically defined chemical reaction permutation within a network. Starting with these chemical reaction modules, we assemble concise mathematical blueprints of microbial metabolisms.
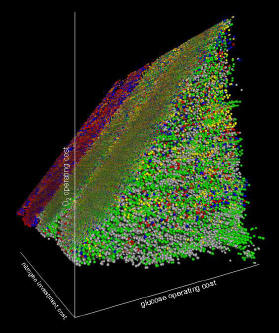
The network analysis research is integrated into practical microbial engineering and microbial physiology studies. Analogous to traditional chemical engineering, we apply an understanding of specific chemistries to control the conversion of inexpensive or undesirable compounds into more valuable or more desirable products. However, instead of using traditional chemical engineering approaches involving inorganic catalysts and high temperatures, we are using molecular biology and metabolic blueprints obtained from network analysis to engineer useful processes catalyzed by microbes. Applications of this work include environmentally critical processes like efficient nutrient recycling and bioremediation of contaminated sites. The research can also be used to optimize biotechnological processes like the production of biofuels and biomaterials from renewable resources. In addition, by advancing our metabolic understanding of microbial pathogens, it should be possible to improve prevention and treatment strategies for medical infections.
Fungal Biomats: Biocatalysts for Producing Protein and Biochemicals
Filamentous fungi can form biomats consisting of a network of closely associated, linearly arranged, hyphae known as mycelium. These biomats are high density biocatalysts capable of using a wide range of inexpensive substrates spanning simple sugars to agricultural waste and can synthesize a variety of valuable products including high quality protein for human consumption or useful biochemicals such as biofuels. The biomats have high volumetric productivities, modest water requirements, and low energy demand because they are not mixed. The biomats exist at the intersection of biotic and abiotic processes due to the fungal requirements for resources which are delivered via transport processes including diffusion and convection.
Figure Courtesy of Dr. Laura Camilleri. 3D reconstruction utilizing z-stacks from confocal laser microscopy depicting a fungal biomat stained with (A) calcofluor white, or (B) nile red. Box (C) is an example of a single 2D image collected from the calcofluor stained fungal biomat, and (D) demonstrates how the z-stack images can be compiled into a space filling model.
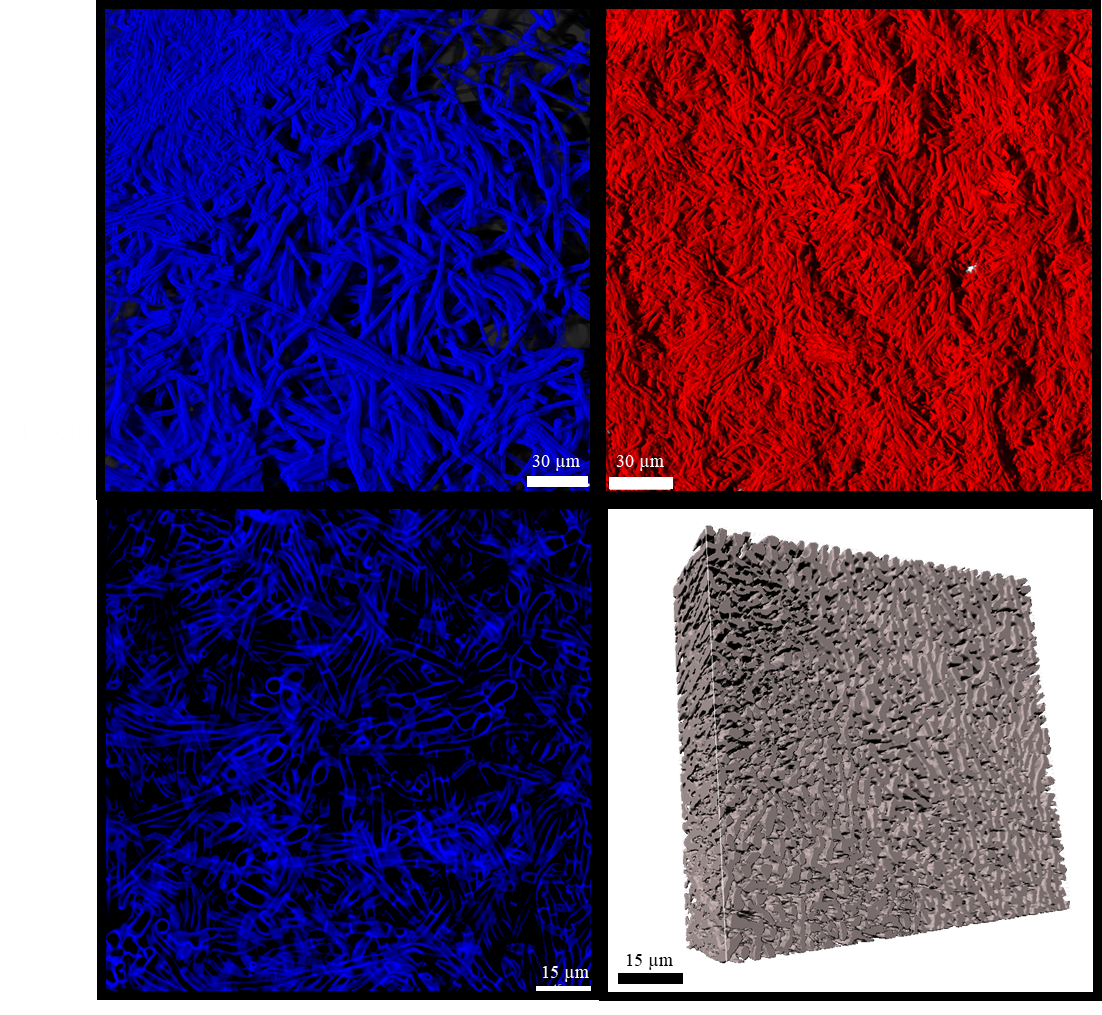
Our research focuses on optimizing fungal biomat physiology to maximize desired properties like growth yields with minimal byproduct secretion. We also quantify spatial heterogeneity within the biomat by analyzing chemical gradients with tools such as oxygen microelectrodes and 3D image analysis of physiological processes like protein synthesis using state of the art microscopy techniques.
Chronic Wound Consortia
Chronic wounds can start as a variety of wound types before becoming classified as chronic after showing little to no signs of healing for an extended period which can be on the order of months, years, or decades. Diabetic ulcers, sickle cell ulcers, vasculitis, and bed sores (pressure ulcers) are some wounds associated with comorbidities and are more likely to become chronic wounds. It is estimated that 15% of diabetic patients will develop lower extremity ulcers and that 14-24% of the patients who develop foot ulcers will undergo amputation. 70.9% of diabetic patients who undergo amputations will have a life expectancy of less than 3 years after amputation.
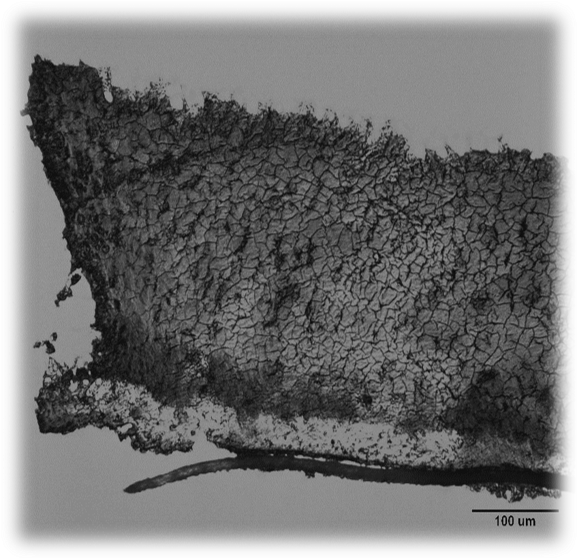 Micrograph of three species colony biofilm. Top to bottom: P. aeruginosa, mixed cells and debris, S. aureus, C. perfringens, carbonate membrane. Image courtesy of Lee McGill.
Micrograph of three species colony biofilm. Top to bottom: P. aeruginosa, mixed cells and debris, S. aureus, C. perfringens, carbonate membrane. Image courtesy of Lee McGill.
Our research focuses on metabolic interactions and 3-D distribution within in vitro biofilms of three bacterial species often co-isolated from chronic wounds: Pseudomonas aeruginosa, Staphylococcus aureus, and Clostridium perfringens. These three species exchange metabolites, and P. aeruginosa and S. aureus create oxygen gradients resulting in anoxic zones suitable for C. perfringens growth. Data acquired from in vitro experiments are used to inform parameters of in silico experiments for more efficient determination of potential treatments for biofilm eradication.
Consortia Engineering
Microalgae for Biochemicals
As the world’s population increases, demand for fuel, food, and manufactured products is also increasing. There is an urgent need for sustainable production of chemical feedstocks for fuel and for nutritional supplements like vitamins and single cell protein. Microalgae have several advantages over other biological sources of these compounds: they utilize sunlight and CO2, which are inexpensive and readily available; they can be grown on non-arable land, so they do not compete with traditional food production; and they grow faster than plants, meaning higher product synthesis rates and faster development of new strains.
We are using metabolic network analysis to investigate the production of multi-carbon compounds from bicarbonate and CO2 by an alkali-tolerant Chlorella microalgaspecies. Our focus is on the synthesis of triacylglycerols, which can be used to make biofuel or polymers, as well as polyamines, which are commonly used in the pharmaceutical and chemical industries. These storage compounds can be over-produced by algae grown under light or nitrogen stress.
Anti-Biofilm Coatings
Biofilms are 3-dimensional microbial communities encapsulated in a self-produced polymeric matrix. The combined physical and physiological properties of biofilms make them very difficult to control. Common planktonic microbial control strategies like the use of antibiotics or oxidizing chemicals are typically limited in their efficacy at inhibiting or removing biofilms. Biofilms can grow on most moist surfaces making them a ubiquitous problem faced by a broad range of disciplines including the medical field. For instance, the National Institutes of Health estimates that up to 80% of human infections are related to biofilms. Implanted medical devices like catheters and artificial joints often serve as a vector for biofilm related infections.
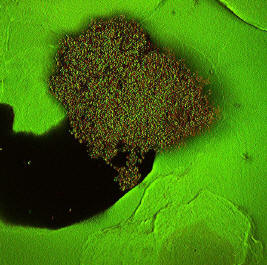
We are studying surface coating strategies for retarding or preventing the formation of biofilms in collaboration with the Center for Biofilm Engineering. The strategies do not utilize the traditional paradigm of antimicrobial agents imbedded in an inert polymeric material. Instead, the system utilizes a naturally occurring polysaccharide as both the coating material and an actual anti-biofilm agent. The coating material possesses inherent, broad spectrum, antimicrobial properties, is biocompatible and is quite non-toxic to mammalian cells. The coatings have been shown to be effective at retarding or preventing the formation of biofilms under medically relevant conditions. The coatings have performed significantly better than more traditional coating systems impregnated with antimicrobial agents like chlorhexidine. The findings suggest this polysaccharide- based strategy has strong potential for applications on surfaces susceptible to biofilm formation.
